|
Technical Problem and Solution
C. Bio-Nano Synthesis Using SPL Techniques for Biotechnology Applications:
The industrial biotechnology standards for genomic and proteomic assays require parallel fluid delivery and experimental testing of 48, 96, 384 or a higher number of unique chemical species. The fluid handling for these operations is usually achieved by a robotic fluid dispenser. For the SPL process to be scaled to the required industrial standards - larger numbers of unique species need to be delivered than are possible by current Inkwell™ devices. However, increasing the throughput of the number of inks creates challenges for fluid metering, delivery, the spatial real-estate on the device and the spatial addressing of the chemical species while compromising reliability and cross-contamination issues. Also, the minimum fluid volume dispensing capabilities of the commercial robotic dispensers are limited to about 1 ml (micro-liter). The SPL process typically requires fluid dispensing volume of 100 pico-liters or less, which is about 10,000 times smaller than the capability of the current robotic dispensers. This makes the SPL technique more challenging and also more attractive due to the significant material savings of costly reagents, probes, primers and analytes used in the assays.
| The proposed study (Figure 6) involves a scheme for synthesizing biological materials (oligonucleotides, peptides, etc.) and maximizing the throughput of the number of inks to conform to the industrial standard of 96 (or higher) for DPN applications in biotechnology. |
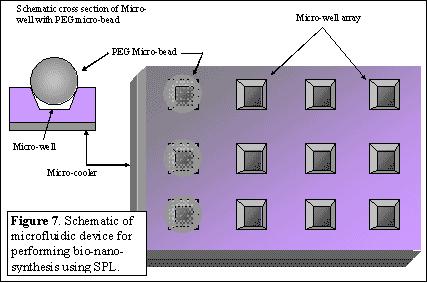 |
The scheme consists of the design, fabrication of microfluidic devices with integrated micro-coolers, and environmental control system. The proposed scheme obviates the need for fluid metering devices, pumping mechanisms and spatial addressing issues and their associated complications. The microfluidic device proposed here consists of a two-dimensional array of micro-wells formed on a substrate. The micro-wells will be integrated with commercially available micro-coolers (thermo-electric coolers, Peltier devices). Micro-beads of hygroscopic material such as PEG (Poly-Ethylene-Glycol) which are available commercially18 can be dispensed into the micro-wells to create a bead-array co-located with the micro-well array. The microfluidic device will be positioned in the DPN stage housed in the environmental chamber. By controlling temperature ramp-up and ramp-down cycles of the micro-coolers and the environmental control apparatus to below the dew-point it is possible to condense water droplets on the PEG beads. The hygroscopic property of the PEG solutions will ensure that the microwells do not dry out by spontaneous evaporation. Commercially available PEG beads pre-mixed with customized functional molecules of interest (genomic species or proteins/peptides) can also be used. The micro-beads are commercially available in various sizes ranging from 1-1000 microns18. Following the formation of PEG solution in the microwell array – the pen apparatus will be dipped into the microwells for loading inks and subsequent bio-nanolithography steps using multiple inks. This will enable the creation of nano-bio-arrays (gene chips, protein chips, etc.) with substantial scaling in biological information content on these devices.
The work involves designing proper environmental control schemes and temperature ramp-cycles of the microfluidic apparatus. The effect of PEG micro-bead sizes on nucleation and condensation will also be studied. The experiments will be optimized for the material synthesis and lithography steps. The deposited biological materials will be characterized by lateral force microscopy and their biological activity will be characterized by performing appropriate assays.
|
|
|
|